| Author | Message | ||
Reepicheep |
Just because the air / fuel mixture is at or near atmospheric pressure about the time the intake valve closes, that means nothing about the conditions that *got* that air fuel mixture into the cylinder in the first place. It's a transient problem, not a static problem. An overly simplistic view would go as follows... I am looking at just one cylinder on our 1200cc 4 stroke Vtwins. 600cc's (one cylinder) of air gets ingested with one revolution. The passage of that air has to start and finish with that one revolution, that is the only time the intake valve is open. Near redline (6800 rpm) the engine is moving 600cc * 6800 = 4080000 cc's of air a minute. Converting that to CFM gives 144 CFM. That is actually a lot less then I expected. That is the simplistic view. The pressure dufferential is not a steady flow, it is a transient. A greater initial pressure differential is created, initiating flow, and that flow lowers the differential until equilibrium is reached. I don't know if this is a linear or exponential, but everything else I can think of in this area is exponential, so this likely is also. So two simple questions (likely with complicated answers) exist. 1) What sort of transient pressure diferential is necessary to get this discrete pulse 144cfm flow to move through a typical Buell carb (1" ?) and intake tract? 2) What is the effect of that pressure differential curve on atomized fuel at normal engine temperatures for a typical Buell intake tract. The unstated third question here is "does it really matter", which is to say is there a significant difference in combustion between an atomized, a partially evaporated, or a fully evaporated air fuel mixture? I don't claim to know the answers, I am just guessing the direction Blake was leading me in. The answers to the above questions would be very interesting to me though, if any mechanical types want to take a crack at it. I lack the background for it, and sold my Thermodynamics textbook to buy pizza for all my EE friends to celebrate the fact that I would never need to see another steam table as long as I live  Bill | ||
Rick_A |
Well, you guys raised some good points that had escaped my mind. Someone had told me once that it's impossible to reach 100% volumetric efficiency in a N/A engine. Some high performance engine builders claim to do just that. The cost is inefficiency lower in the power curve, though. Exhaust and intake momentum both contribute to scavenging. The sound waves (reversion) of the exhaust play a more important role as well. They are tuned for the purpose of returning the intake charge drawn out the exhaust back into the cylinder in high overlap engines...but only to a limited range. The Yamaha EXUP valve was designed to improve efficiency by altering the timing of that wave to be useful at all RPM and therefore keep more of the mixture in the combustion chamber and give a broader power band despite wide overlap. I'm surprised this kind of technology hasn't been used more. It's similar to the effect of a power valve in a two stroke. | ||
Sparky |
When fuel lines get too hot, vapor lock can occur -- the fuel boils and interrupts fuel flow. That's probably why our carbed Buells have thermal insulated sleeves on the gas hoses. | ||
Hootowl |
Rick, I know that 100% is a theoretical maximum, which is why I was hesitant to say that you can exceed it. But at the same time, I read articles in Hot Rod and other motor mags that claim to do just that. Hmmm. | ||
Rempss |
The Denish books mention VE up to 130% on a Harley V-twin motor. I cannot tell you the exact context, I do not have the books here. But it's referenced several times in the 3 book series. Jeff | ||
Jim_Witt |
Jeff, I'm not the expert but I thought cooling the fuel in drag racing is used to help stabilize the fuel pressure and prevent vapor lock? With the overwhelming technology available, along with the precise calculations that are necessary to be completive, getting everything physically setup correctly within a particular environmental condition, to mimic the ideal computer simulation, is critical. Stabilizing the fuel is just one item on their checklist. I'll also assume they "try" to cool the intake manifold to make the air denser and richer in oxygen, similar to what my innercooler trys to do on my turbocharger. Dunno, -JW:>  | ||
Hootowl |
Jim, That makes sense. I wasn't saying WHY they do it, I was asking. | ||
Jim_Witt |
Bill asked: The unstated third question here is "does it really matter", which is to say is there a significant difference in combustion between an atomized, a partially evaporated, or a fully evaporated air fuel mixture? So is your post above (the unstated 3rd question) somewhat of a summery of what started all of this so called debate? If so, I'll change my guess and say YES. I'd say a "fully vaporized fuel" condition (not atomized) adds a significant change in combustion efficiency. S'later, -JW:>  | ||
Hans |
Another consideration: Assume the well known V-twin makes 80 HP at 6000 RPM with it cylinders full of the ideal mix. Slow tickling at idling at 1200 RPM, 1/5 of the RPM, with its cylinders again full of the same ideal mix it would raise 16 HP. While the starter motor is 2000 Watt and turns an hot engine at about 800/1000 RPM it sounds reasonable that 4 HP or even less is needed to keep the engine idling. It will burn then about 1000 gram/hour: also a acceptable value. The engine is well tuned and also at idling it burns an ideal mixture, but now it needs only 1/4 of the volume to raise 4 HP needed for steady idling. This means that an idling engine should have in its cylinders 1/4 of the fresh air. And while the displacement of the piston keeps the same: The pressure of that fresh air has to 1/4 of the athmospheric pressure. Oh, Now I understand why that slid at closed throttle is so very small: engine has to be choked to death almost, letting only 1/4 of the fresh air in. And what a pressure differential. Hans That is not needed: it would speed up and fast. | ||
Blake |
Rick, You are so adamanst that I "prove" my statements. Why don't you "prove" that vaporization does NOT occur in a significant way within a carburetor? Shoe on the other foot doesn't fit to well does it? My point has not changed. In *trying* (so unsuccessfully) to lead you to understand how gasoline can vaporize within a carburetor, I started with what I thought was the MOST simple, logical and easily understood example, that if gasoline under significant pressure within an injector can vaporize, then obviously gasoline being sprayed/atomized into a stream of lower pressure air would also vaporize. You could not see that. Fine, I continued on another track, less intuitive. You do accept that by evaporative cooling, the air fuel mixture passing through the carburetor can cool by as much or more than 30oF below the ambient temperature. However, you do not (or should I say "will not"  ) recognize how that significant amount of cooling indicates in turn that a significant evaporation of gasoline must transpire within the carburetor. There is a simple way to check that. It is called thermodynamics. ) recognize how that significant amount of cooling indicates in turn that a significant evaporation of gasoline must transpire within the carburetor. There is a simple way to check that. It is called thermodynamics. To make the analysis simple and easy to understand for you, we need to make some conservative assumptions, conservative in that they will not significantly affect the validity of our result and in fact will tend on the whole to reduce the magnitude of the result that we are seeking to calculate... Problem: For A/F=15, determine the maximum possible drop in temperature due solely to the effect of vaporizing gasoline. Assumptions: Dry air (zero humidity), Constant Pressure (no pressure drop due to venturi or otherwise, no heat added to or removed from the mixture (a perfectly insulated container). Given: Subscripts "g" and "a" indicate gasoline and air, respectively. Superscripts "L" and "V" indicate liquid and vapor, respectively. Pressure (P) is constant at 1.0 ATM = 14.7 PSI Initial Temperature (T1)=80oF Specific Heat of Gasoline Liquid (CpgL)=0.50 BTU/LBmoR Specific Heat of Gasoline Vapor (CpgV)=0.40 BTU/LBmoR Heat of Vaporization of Gasoline (hVg)=150 BTU/LBm Specific Heat of Air (Cpa)=0.24 BTU/LBmoR Relative Humidity = 0% The law of conservation of energy states that energy can be neither created nor destroyed, only transferred and/or transformed. In Thermodynamics that basic law of science is dubbed the "First Law of Thermodynamics" In other words, if we do not remove energy from or add energy to an isolated system, that system will have no change in its total "internal" energy. That is why we specified that the system under analysis is isolated from energy influences other than its own; or a perfectly insulated carburetor in our case. By doing so, we are saying that we are not letting any energy in or out of the air/fuel flow. You may wonder how the temperature can decrease with no change within a thermodynamic system's total energy. It is simple really, a substance in a gaseous state has a lot more energy than when in liquid state which has a lot more energy than when in solid state. So water vapor at room temperature will have more energy than an equal mass of liquid water at 200oF. The molecules in gaseous form are highly energized and fly around all over, bouncing off of each other. As a liquid they wiggle around a bit. As a solid, they quiver. Of coures of two samples of matter in the same state, the one with the higher temperature contains more energy. The above applies under constant pressure. You can find more information in a high school physics and/or chemistry book if you are interested in phase change thermodynamics and the implications here. So anyway, we have specified an isolated system, or more precisely a steady flow system in which mass entering equals mass exiting, and between the entrance and exit all pertinent conditions remain steady (inlet temperature and pressure remain the same while the exit temperature and pressure also remain constant) and we are not adding or removing energy between the inlet(s) and outlet. We have three forms of matter to consider in our analysis, air, liquid gasoline, and gasoline vapor. We know the ratio of the mass of the air to the mass of the gasoline (liquid + vapor) because we specified it to be the typical 15:1 EPA friendly air/fuel ratio. What we want to determine is the total cooling effect that the liquid gasoline provides in such a ratio as it transforms to a gaseous state (vaporizes). As stated before, the heat (Q) required to raise the energy of the liquid gasoline enough to transform it into a vapor comes from the ambient air. So the air is loosing heat and the liquid gasoline is gaining heat. The gasoline vapor, as it cools also loses heat. So for conservation of energy we have... or QGained + QLost = 0 or simply... DQ =0 A substance's gain or loss of heat energy under constant pressure can be calculated from the equation... or Q=m*Cp*DT where "m" is the mass of the substance of interest. The heat required to vaporize a liquid (heat gained by the liquid) is found from the relation... where the terms are as previously defined above. From the above we can write QGained=mghgV QGained=1*(150)=150 BTU QLost=-maCpaDT-mgCpgVDT QLost=-15*0.24DT-1*0.40DT QLost=-4.0DT Inserting the above into... DQ =0 Yields... DQ=0=150-4.0DT solving for DT gives us... DT=37.5oR=37.5oF Summarizing: If allowed to evaporate entirely the liquid gasoline comrising a 15:1 F/A mixture will reduce the temperature of the mixture by approximately 37.5oF. Is it obvious to you now that if the air-fuel charge flowing through the carburetor is cooled by, as you yourself contend, more than 30oF, that a significant amount of gasoline must indeed be vaporizing? I mean since if the entire fuel charge were to evaporate within the carburetor, for an ambient temperature of 80oF the temperature would only decrease by 37.5oF. I also performed a quick check on that using another method, very chart intense, not possible to demonstrate here. The answer so obtained checked out. If you go through a similar exercise but instead of solving for DT, you set DT=20oF and solve for the mass of vaporized gasoline you will find that for a 15:1 A/F ratio, almost half of the gasoline needs to evaporate to drop the air-fuel charge temperature by 20oF. On atomization... It happens within a very short time and distance inside the carburetor. Once the atomized fuel droplets accelerate to near the speed of the airflow and/or achieve a stable size droplet, further atomization ceases. That happens within a very short distance within the carburetor. On some of your sources... not what I would call reputable or reliable. Hucksters peddling their wares with a disingenuous sales pitch. You trust them over me? Nice. Try Chevron's Gasoline Technical Information Site. Page almost halfway down to the "Carburetor Icing" section. They say there, as had already been offered by other here, that carb icing can be a serious factor at temperatures up to 55oF. That'd be 23oF above the freezing point of water in ambient conditions. At a lower pressure, like that actually found on the engine side of the throttle plate, it will freeze at a higher temperature than 32oF. The dew point for ambient air at 50%RH and 80oF is 64.4oF. At that temperature, inside the carburetor the water vapor begins to condense from gaseous state to liquid state. That process liberates heat since the water is falling back to a less energetic state. Make sense? Of course we neglected the pressure drop and the presence of humidity. The pressure drop would help to facilitate vaporization. Humidity, if brought to the point of condensation would help to facilitate evaporation too, as it releases heat. So, what did you finally learn from all this? Now that you have your proof, are you satisfied? | ||
Blake |
Hans, That's a very good point, 2,000W=2.68HP. Are you sure the starter is 2KW? That'd pull over 150 amps! I can't imagine needing more than 1/2 hp to keep the engine pumping at idle. Plus the starter is on a 15A fuse is it not? So the max power would be less than 12V*15A=180W which would be 0.24 HP. Sounds about right to me. I once averaged 74 mpg on two consecutive tanks when traveling lazily along at 70 mph in colorado. The 74 mpg at 70 mph equates to 70/74=0.946 gal/hr. Let's use round numbers and just say 1 gallon per hour. At 70 mph my M2 was turning about 3,400 rpm compared to 1,000 rpm at idle, so the fuel rate at idle would be no more than 1,000/3,400*0.946=0.278 gph, an upper bound. Knowing that it takes about 75 rwhp for my M2 to peak out at 120 mph (personal empirical data) and knowing that the RWHP required to propel a vehicle through air is proportional to the speed cubed we can estimate that the power required to propel me on my M2 at 70 mph is 0.85*75(70/120)3=12.65 RWHP. The 0.85 is the altitude correction factor for drag in the thinner air up there. Taking the starter power of 0.24HP we can say that the fuel consumption at idle would be somewhere around 0.24/12.65*1 gal/hr=0.019gal/hr fuel consumption and so with a A/F ratio of around 13 the engine would be drawing 15*0.019gal/hr*6.4LB/gal=1.824 LB/HR=0.0304LB/min of air. At 1,000 rpm the engine is trying to take in 1,000/2*73.2CI/1782CI/CF=20.5 CF/min which at STP would amount to 1.44 LBm/min of air. So with the throttle closed at idle the engine is seeing a VE=.0304/1.44=0.021=2.1% That's a pretty strong vacuum. To check, calculate the A/F ratio for the 70mph at 3,400 rpm and 1 gal/hr fuel rate. Assuming VE=1.0 (wild ass guess or WAG)  , spinning at 3,400 rpm for an hour would give us (3,400/2)*60= 102,000 intake cycles for a total intake air volume of ... 102,000*73.2CI/1782CI/CF=4323 cubic feet (CF). , spinning at 3,400 rpm for an hour would give us (3,400/2)*60= 102,000 intake cycles for a total intake air volume of ... 102,000*73.2CI/1782CI/CF=4323 cubic feet (CF). At STP a cubic foot of air contains 0.07651 LB mass of air. So the engine would have taken in 4,323CF*0.07651LB/CF=330.7 LBm of air. Back to the case of cruising at 70mph and 74 mpg. One gallon weighs around 6.4 LB so the A/F ratio would come up shy at 330.7/6.4=51.7:1. That's a bit lean by a factor of 51.7/15=3.4 which indicates that with the throttle at a cruising at 70 mph position the volumetric efficiency is only around 1.0/3.4=0.29. That's quite a vacuum eh Rick? Would you believe me if I said I had all that typed up last week? Was waiting for Rick to make a breakthrough.  | ||
Rick_A |
I figured out how I was screwed up here...the text I have seems to have the flow of air described as it would be for a liquid. I don't know if that was for the sake of simplicity but it certainly screwed me up. So I realize now that here is indeed a vacuum...but, once the intake valve closes the inertia of this column of air/fuel compresses against it. How's that factor in? | ||
Blake |
If the intake tract geometry is properly configured, it contributes to a ram induction effect, similar but not quite as effective as the scavenging that can be accomplished on the exhaust end. The pressure pulse bounces back and forth between intake tract inlet and intake valve. If the engine speed is right and the geometry is right, the pressure pulse arrives back concurrently with intake valve opening. Conversely, if the pulse arrives at the intake tract's inlet concurrent to valve opening, there is a vacuum pulse at the intake valve that impedes cylinder fill. Pretty much the same as the exhaust except reversed. Exhaust wants a vacuum pulse at the exhaust valve as it is still open and intake valve is opening. Intake wants a pressure pulse. The mistake you made was to assume the flow was incompressible. Your reference to Bernoulli gave that away along time ago. That applies to most liquids and to steady flow of ideal (or close to it) gases for flow below Mach 0.4. The flow in a carburetor is neither liquid, steady, nor always below Mach 0.4. That mistaken assumption relates to the vacuum debate. It has no significant effect on the vaporization phenomenon as that would occur to similar extents for steady or unsteady flow just the same. The equations describing compressible, unsteady, viscous flow with heat gains (radiation from engine, convection on cold carb body) are WAY beyond anything we can cover here. Like I said before, this actually IS rocket science. | ||
Jim_Witt |
Carb 101 Who knows, some of you may find this interesting reading, I did. Cheers, -JW:>  | ||
Rick_A |
This is about the best graphic illustration to CV carb tuning I've found: 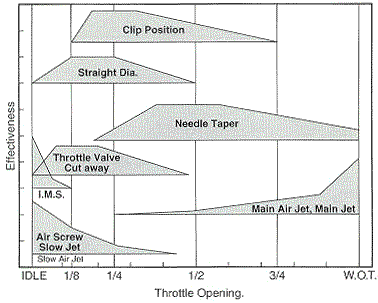 | ||
Rick_A |
I haven't been able to figure out what I.M.S. refers to. | ||
Aesquire |
Whew! great if heated discussion. Vaporizing the fuel before the carb is the trick used in the Pouge carb. Fuel goes thru a heat exchanger then is metered (poorly in the example I saw) into the throat of the carb as a vapor. There are some serious safety issues with the Pouge carb as you are dealing with hot vaporized fuel. (see fuel air bombs lol) My pals VW had a kludged setup that required you to turn off the fuel to the heat can & run the engine until the fuel in the can had all vaporised, otherwise you had nifty blowtorch carb fires (yep trial & lots of error) in his defense, he went to a seminar on the Pouge & never had real plans. | ||
Aesquire |
here's a page (first try w/google) full of paranoid energy comments http://www.fortunecity.com/greenfield/bp/16/feedback.html | ||
Aesquire |
Smokey Yunick's setup seemed to avoid the problems of heating the fuel by compressing the mixture with a small low boost turbo. Without re designing the whole engine, I think the traditional "cool the mix" wisdom is still best. My drag racing friends 440 Charger can start, & run indefinetly at idle (with testosterone pleasing blips) with a cool to the touch intake manifold. Shut the engine down & you can feel the manifold heat up from conduction very rapidly. (560 HP low 11's in the 1/4) | ||
Jim_Witt |
 | ||
Blake |
"I haven't been able to figure out what I.M.S. refers to." I'd tell you, but you'd probably defame me and insist that I prove it.   | ||
Hans |
Blake, The 2000 Watt power of the starter motor came out of my thumb, but not completely: My Laverda 750 had the same starter as a VW beetle. Those are 1200 or 1600 Watts if I remember well. The Maserati pulled 350 Amps from the battery at the start told me the mecanicien. I suppose the 15 Amp fuse is only protecting the starter circuit to the relais. Starter motor itself is connected directly via the relais to the battery. Well, you picked up my point very well. Thank you very much for your explanations. Can`t get enough from that stuff. Hans | ||
Sarodude |
Isn't IMS Idle Mixture Screw? Also, isn't that more for a straight flat slide? They're referring to throttle position as opposed to rev ranges. -Saro | ||
Rick_A |
Throttle position and rev range are pretty much one in the same. Rev range isn't a practical representation for a guide intended for all applications...and yeah...it must be idle mixture screw. I found the chart on a CV tuning page...but a flat slide has the same adjustability. The difference is that the CV uses a throttle plate and a slide dependent on venturi signal rather than mechanical control. | ||
Rick_A |
Exhaust scavenging works more to evacuate the cylinder than positively fill it, wouldn't it?...It "sucks" rather than force mixture in. It seems to me that the intake charge and the exhaust return pulses work more to positively fill the cylinder. | ||
Blake |
"Throttle position and rev range are pretty much one in the same." Really? Is the fuel delivery circuit at 25% throttle/5,000 rpm the same as for WOT/5,000 rpm or is it the same as for WOT/3,000 rpm? That graphic is excellent for steady speed (little acceleration happening) applications. It could give some the impression that if they wack the throttle wide open at low revs and the engine runs poorly, that they would need to adjust the main jet. That would be wrong. What is needed for a truly comprehensive treatment is a three dimensional chart with engine speed as % of rev limit as the Z-axis. | ||
Rick_A |
It also depends on the engine...engines with no or little overlap have less effect in regards to exhaust tuning. | ||
Rick_A |
really? Is the fuel delivery circuit at 25% throttle/5,000 rpm the same as for WOT/5,000 rpm or is it the same as for WOT/3,000 rpm? Well, that's pretty irrelevant. For one, you still have to go through all the remaining circuits when you wack the throttle regardless...so if you tune all circuits well from idle to WOT they should all function well at WOT from any point. Even in a dyno run with EGA, at the short duration that it is, and being at WOT, effectively shows the F/A ratio though the rev range. You still go through all the circuits just quicker. Trying to tune by WOT from partial throttle openings seems pretty useless to me. It could give some the impression that if they wack the throttle wide open at low revs and the engine runs poorly, that they would need to adjust the main jet. That would be wrong. Not if you think of your motor's approximate rev range in relation to the chart. It's a basic guide...and I think it's helpful in that respect. | ||
Blake |
"Exhaust scavenging works more to evacuate the cylinder than positively fill it, wouldn't it?...It "sucks" rather than force mixture in." I believe that is an accurate characterization of exhaust scavenging. "It seems to me that the intake charge and the exhaust return pulses work more to positively fill the cylinder." That would be accurate for the intake charge where a pressure pulse aids better cylinder filling. A greater pressure differential across an intake valve improves rate of flow into the cylinder and thus improves cylinder fill (volumetric efficiency or VE). An undesired positive pressure pulse from the exhaust side would try to reverse (as in reversion) fill the cylinder. Aside from the detrimental effects of possibly adding exhaust to the intake charge, the exhaust pressure pulse is stronger than the intake pulse, so it acts to push intake charge back out of the cylinder; when the exhaust valve finally closes, the intake charge must then change directions and get going back towards the cylinder. The idea behind efficient cylinder fill (VE) is to keep the momentum of the fluid wrt the combustion chamber always moving in the same direction, into the intake port and away from the exhaust port. Exhaust reversion does the opposite causing the intake charge to stop, reverse, stop again, then start back towards the cylinder. That takes energy; the intake charge is the donor. Less energy in the intake charge means less efficient cylinder fill. Not good. The negative exhaust pressure pulse or vacuum pulse working to scavenge or suck the intake charge into the combustion chamber adds energy to the intake charge. A good thing. Think of it as two guys pulling a load with a rope. If they pull in the same direction things seem to go better than if one pulls in the opposite direction of the other. | ||
Blake |
Like I said...  That graphic is excellent for steady speed (little acceleration happening) applications. Rick, I'm not picking on you personally, really. You bring a lot to this forum and I appreciate your enthusiastic contributions. Please realize that when I read posts offering as fact statements technical in nature, especially here in the knowledge vault, I try to review them with the rigorous eye of a technical editor. To do so I assume that the audience is comprised of those who know little about the subject, that they are largely ignorant of the topic. If clarification is necessary, I offer it or try to induce the original author to do so himself. I do not want to start an argument every time someone posts something that requires clarification. We should all carefully consider our statements and whether or not we are communicating accurately and comprehensively the facts. We should demand that from everyone here, me included. Now we all make mistakes, I certainly do. When a clarification is offered or correction provided, we might be better off to offer thanks rather than engage in a battle of egos. Cool? |